

Mitochondrial Dysfunction: The Role of Nutritional Supplementation
The Relationship Between Autism and Mitochondrial Dysfunction
Mitochondrial disorders
While the exact causes of autism are still unknown, researchers have recently uncovered a mysterious link between autism and mitochondrial disorders. Mitochondrial disorders are caused by mutations in the mitochondria, which are the parts of our cells that produce energy, and can lead to a wide range of symptoms. This link has made it possible to identify and treat some cases of autism, offering hope to those affected by this condition. In this blog post, we’ll discuss the intriguing connection between autism and mitochondrial disorders and what this could mean for those living with autism.
Mitochondria
Mitochondrial disorder
Mitochondrial disorders are a group of complex and potentially life-threatening illnesses caused by mutations in the mitochondrial DNA. Mitochondria are tiny organelles found inside our cells that play a key role in energy production. They convert energy from food into a form that our bodies can use to function normally.
Sympoms of mitochondrial dysfunction
The most common symptoms are fatigue, but brain fog is also common when the mitochondria in the brain are not working properly.
Other symptoms may include:
- pain
- mood disorders
- anxiety
- depression
- concentration disorder
Most chronic diseases/conditions are also linked to mitochondrial dysfunction, such as:
- cardiovascular diseases
- lung disease
- vision and hearing problems
- learning disabilities
- autism
- liver and kidney disease
- digestive system diseases and symptoms
- diabetes
- neurological diseases (including dementia)
- movement disorders
The Hannah Poling affair
If the child has a mitochondrial enzyme deficiency, the vaccines can cause developmental declines.
Biomedical specialists believe that mitochondrial problems can be very common in autism; however, there are treatments that can improve mitochondrial disorders.
It would be nice if mitochondrial testing could become part of normal neonatal screening to find out which babies may be more sensitive to heavy metals and chemicals — whether they have “only” mitochondrial dysfunction or mitochondria are functioning completely abnormally — and preventive measures could be taken to protect children.
If the child has a mitochondrial enzyme deficiency, the vaccines can cause developmental declines.
Biomedical specialists believe that mitochondrial problems can be very common in autism; however, there are treatments that can improve mitochondrial disorders.
It would be nice if mitochondrial testing could become part of normal neonatal screening to find out which babies may be more sensitive to heavy metals and chemicals — whether they have “only” mitochondrial dysfunction or mitochondria are functioning completely abnormally — and preventive measures could be taken to protect children.
Related articles:
- Mitochondrial disorder and autism
- Supplements for mitochondrial disorders
Resources
- https://www.sciencedirect.com/science/article/pii/S2352304220300854
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5137782/

Omega 3 fatty acid
Omega-3 fatty acids belong to the group of polyunsaturated fatty acids, of which EPA (eicosapentaenoic acid), DHA (docosahexaenoic acid), and alpha-linolenic acid (ALA) are best known. Omega-3 has many properties that help maintain our health, and are an essential bioactive ingredient for children.
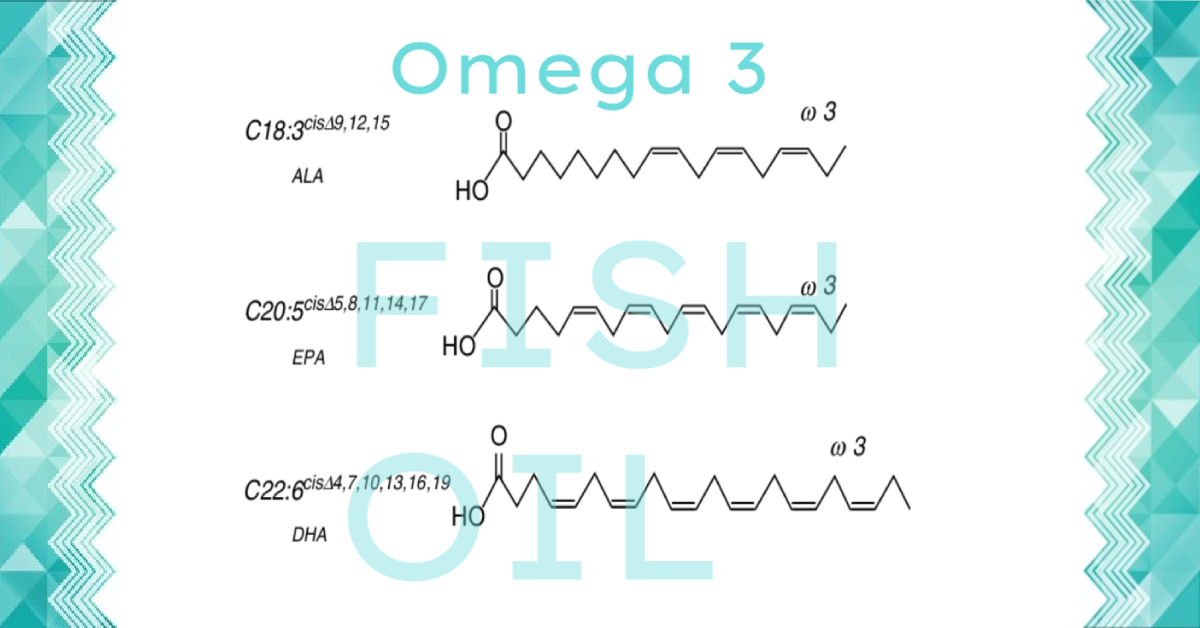
Omega 3 is mainly an anti-inflammatory agent.
EPA has a strong anti-inflammatory effect and not enough DHA in the diet may reduce the ability to handle sensory input. It only takes a small decrement in brain DHA to produce losses in brain function. (1)
In humans, weak sensorimotor gating is a hallmark of many nervous-system disorders such as schizophrenia or ADHD. Given mounting evidence of the role omega-3s play in the nervous system, there is intense interest in their therapeutic potential, perhaps as a supplement to medicines. For example, people with schizophrenia have lower levels of essential fatty acids, possibly from a genetic variation that results in poor metabolism of these nutrients.
The finding connects low omega-3s to the information-processing problems found in people with schizophrenia; bipolar, obsessive-compulsive, and attention-deficit hyperactivity disorders; Huntington’s disease; and other afflictions of the nervous system.
Omega 3 fatty acid
Omega 3 fatty acid belongs to the group of polyunsaturated fatty acids, of which EPA (eicosapentaenoic acid), DHA (docosahexaenoic acid), and alpha linolenic acid (ALA) are best known. Omega 3 is an essential fatty acid.
Consumption of much marine fish is no longer recommended today due to the pollution of the seas. The most practical solution for consuming EPA, DHA, and GLA is by using high-quality fish oil, borage oil, or evening primrose oil in encapsulated form.
Main physiological effects of histamine intolerance and intestinal problems:
In terms of histamine intolerance, its main effect is its ability to reduce stress and reduce inflammation.
It also helps prevent the development of insulin resistance and has a positive effect on weight loss by helping to break down fat cells.
Physiological effects of Omega 3:
- Due to their anti-inflammatory effect, it can reduce the risk of many diseases, such as vascular disease, Alzheimer’s disease, and some cancers. But fatty acids can also help treat diabetes, high blood pressure, and skin conditions.
- Proper intake is essential for athletes due to the maintenance of the cardiovascular system, but it can also improve respiratory function and prevent asthma.
- It helps the development of the fetus and reduces the incidence of preterm birth. It has a role in developing good visual acuity and mental development in infants.
- Lower blood cholesterol levels, reducing the risk of atherosclerosis and heart attack.
- It plays a role in the transport of fats, and the formation of anti-inflammatory prostaglandins, and their beneficial effects on the immune system are also known. And are involved in the structure of the nervous system and cell membranes.
- Recent research has shown that children consuming omega-3 fatty acids regularly have improved their task-solving and problem-solving skills to understand tasks faster and improve school performance. Achieving optimal intake by children up to 14 is extremely important, as children’s brains develop to the greatest extent by the time they reach this age.
- Omega 3 prevents the development of kidney stones
Other benefits of fish oil according to health problems:
Aging processes
Slows down the aging process.
Cardiovascular system
- counteracts arrhythmia symptoms.
- prevents tachycardia.
- makes it easier to prevent atherosclerosis.
- Prevents the development of cardiovascular disease.
- improves heart function.
- reduces elevated hematocrit levels.
- lowers blood pressure in hypertensive patients.
- reduces the development of Raynaud’s disease.
Digestive system
- reduces the number of relapsing Crohn’s diseases.
- prevents the formation of gallstones.
Eyes / vision
- Fish oil prevents the development of macular degeneration (ARMD) in old age.
- In the case of dry eyes, fish oil is effective in tear production.
- In people with wolf blindness, fish oil can improve vision in the dark.
- Fish oil is easy for the development and treatment of retinopathy.
Immune system
- Children of women who consume fish oil during pregnancy have a lower rate of allergy.
- Regular consumption of fish oil to avoid the development of autoimmune diseases.
- Fish oil prevents the development of certain cancers.
- It has an anti-inflammatory effect on fish oil.
Metabolism
- enhances athletic performance.
- reduces the absorption of cholesterol. Fish oil protects muscle fibers from toxic effects (catabolism).
- prevents tired formation.
- prevents the development of insulin resistance.
- helps break down adipose tissue.
- lowers triglyceride levels.
Musculoskeletal system
- reduces back pain.
- promotes bone formation.
- protects the muscles from the breakdown (catabolism).
- Fish oil is an option for the treatment of polymyalgia rheumatica.
- relieves the symptoms of rheumatoid arthritis.
Nervous system
- reduces aggression.
- have a beneficial effect on the behavior of patients with hyperactivity.
- prevent the development of Alzheimer’s disease.
- relieves depression.
- has the potential for recovery after head injuries.
- improves learning ability.
- improves memory.
- relieves migraine symptoms.
- has the potential to relieve the symptoms of Multiple Sclerosis.
- is commonly used in the development of Parkinson’s disease.
- is used for schizophrenia.
- may be suitable for recovery from spinal cord injuries.
- alleviates the toxic effects of mental stress.
Respiratory system
- may be useful in the treatment of acute respiratory distress syndrome (ARSD).
- improves respiratory function in asthmatic patients
- prevents chronic lung disease.
- easily prevents the development of bronchitis.
- prevents emphysema.
- easily improves lung function and reduce the risk of damage from smoking.
Hormanal problems
- easily relieves menstrual cramps.
- reduces the heat waves associated with menopause and relieve depression.
- reduces the risk of developing male infertility.
Skin
- reduces acne sufferers.
- relieves the symptoms of eczema.
- relieves itchy skin.
- improves skin elasticity.
- easily prevents sunburn in sensitive people.
- speeds up wound healing.
Resources
[1]
Wijendran V, Huang MC, Diau GY, et al. Efficacy of dietary arachidonic acid provided as triglyceride or phospholipid as substrates for brain arachidonic acid accretion in baboon neonates. Pediatr Res 2002;51:265-272.
[2]
Goustard-Langelier B, Guesnet P, Durand G,et al. n-3 and n-6 fatty acid enrichment by dietary $sh oil and phospholipid sources in brain cortical areas and nonneural tissues of formula-fed piglets. Lipids 1999;34:5-16.
[3]
Maki KC, Reeves MS, Farmer M, et al. Krill oil supplementation increases plasma concentrations of eicosapentaenoic and docosahexaenoic acids in overweight and obese men and women. Nutr Res 2009;29:609-615.
[4]
Bunea R, El Farrah K, Deutsch L. Evaluation of the effects of Neptune Krill Oil on the clinical course of hyperlipidemia. Altern Med Rev 2004:9:420-428.
[5]
Sampalis F, Bunea R, Pelland MF, et al. Evaluation of the effects of Neptune Krill Oil on the management of premenstrual syndrome and dysmenorrhea. Altern Med Rev 2003;8:171-179.
[6]
Deutsch L. Evaluation of the effect of Neptune Krill Oil on chronic inflammation and arthritic symptoms. J Am Coll Nutr 2007:26:39-48.
[7]
Chang JP, Chen YT, Su KP. Omega-3 polyunsaturated fatty acids (n-3 PUFAs) in cardiovascular diseases (CVDs) and depression: Cardiovasc Psychiatry Neurol 2009;2009:725310. Epub 2009 Sep 27.
[8]
Breslow J. n-3 fatty acids and cardiovascular disease. Am J Clin Nutr 2006;83:1477S-1482S.
[9]
Calzolari I, Fumagalli S, Marchionni N, DiBari M. Polyunsaturated fatty acids and cardiovascular disease. Curr Pharm Des 2009;15:4094-4102.
[10]
No authors listed. Phosphatidylcholine. Altern Med Rev 2002;7:150-154.
[11]
Naguib YM. Antioxidant activities of astaxanthin and related carotenoids. J Agric Food Chem 2000;48:1150-1154.
[12]
Tso P, Drake DS, Black DD, Sabesin SM. Evidence for separate pathways of chylomicron and very low-density lipoprotein assembly and transport by rat small intestine. Am J Physiol 1984;247:G599-G610.
[13]
Amate L, Gil A, Ramirez M. Feeding infant piglets formula with long-chain polyunsaturated fatty acids as triacylglycerols or phospholipids influences the distribution of these fatty acids in plasma lipoprotein fractions. J Nutr 2001;131:1250-1255.
[14]
Tandy S, Chung RW, Wat E, et al. Dietary krill oil supplementation reduces hepatic osteatosis, glycemia, and hypercholesterolemia in high-fat-fed mice. J Agric Food Chem 2009;57:9339-9345.
[15]
Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th ed. New York, NY: Garland Science; 2002.
[16]
Else PL, Hulbert AJ. Membranes as metabolic pacemakers. Clin Exp Pharmacol Physiol 2003;30:559-564
[17]
Kidd PM. Omega-3 DHA and EPA for cognition, behavior, and mood: clinical findings and structural-functional synergies with cell membrane phospholipids. Altern Med Rev. 2007 Sep;12(3):207-27.
[18]
Konagai C, Yanagimoto K, Hayamizu K, Han L, Tsuji T, Koga Y. Effects of krill oil containing n-3 polyunsaturated fatty acids in phospholipid form on human brain function: a randomized controlled trial in healthy elderly volunteers. Clin Interv Aging. 2013;8:1247-57.
[19]
Ebrahimi M, Ghayour-Mobarhan M, Rezaiean S, et al. Omega-3 fatty acid supplements improve the cardiovascular risk profile of subjects with metabolic syndrome, including markers of inflammation and auto-immunity. Acta Cardiol. 2009 Jun;64(3):321-7.
[20]
Derosa G, Cicero AF, Fogari E, et al. Effects of n-3 PUFAs on postprandial variation of metalloproteinases, and inflammatory and insulin resistance parameters in dyslipidemic patients: evaluation with euglycemic clamp and oral fat load. J Clin Lipidol. 2012 Nov-Dec;6(6):553-64.
[21]
Spencer M, Finlin BS, Unal R, et al. Omega-3 fatty acids reduce adipose tissue macrophages in human subjects with insulin resistance. Diabetes. 2013 May;62(5):1709-17.
[22]
Yan Y, Jiang W, Spinetti T, et al. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity. 2013 Jun 27;38(6):1154-63.
[23]
Valensa. FlexPro MD Clinical Trial Overview and Results. (Data on File.) 2011
[24]
McCann JC, Ames BN. Is docosahexaenoic acid, an n-3 long-chain polyunsaturated fatty acid, required for development of normal brain function? An overview of evidence from cognitive and behavioral tests in humans and animals. Am J Clin Nutr 2005;82:281-295.
[25]
Stevens LJ, Zentall SS, Abate ML, et al. Omega-3 fatty acids in boys with behavior, learning, and health problems. Physiol Behav 1996;59:915-920.

